ศาสตร์พระราชาหัวข้อพอเพียง
วันพฤหัสบดีที่ 19 กันยายน พ.ศ. 2562
วันพฤหัสบดีที่ 12 กันยายน พ.ศ. 2562
วันจันทร์ที่ 2 กันยายน พ.ศ. 2562
วันจันทร์ที่ 26 สิงหาคม พ.ศ. 2562
วันจันทร์ที่ 8 กรกฎาคม พ.ศ. 2562
สมดุลเคมี
back to homepage
aA+bB⇌k−1k1cC+dD(1)
rate=−k1[A]a[B]b=k−1[C]c[D]d(2)
K=acCadDaaAabB at equilibrium(3)
Q=acCadDaaAabB at any point in the reaction(4)
dGr=dG∘r+RTlnQ(5)
Chemical equilibrium is a state in which the rate of the forward reaction equals the rate of the backward reaction. In other words, there is no net change in concentrations of reactants and products. This kind of equilibrium is also called dynamic equilibrium.
Introduction
Given the following elementary reaction:
the rate of the forward reaction can be represented by the following equation:
where k1 and k-1 are the reaction constants for the forward and reverse reactions, respectively. Note: the constant k can vary among different reactions at different temperatures.
When the rate is zero, the net concentrations of A, B, C, and D are in equilibrium with each other. Thus, if there is a change in the system due to changes in concentration, temperature, or pressure, the equilibrium will shift to offset the change and re-establish equilibrium according to Le Chatelier's principle. Le Chatelier's principle indicates whether more reactants or more products will be made. Note that the presence of a catalyst does not shift the equilibrium; it only causes the reaction to reach equilibrium faster. This is due to the fact that catalysts only lower activation energies.
Dynamic equilibrium is useful in predicting whether the forward or reverse reaction is spontaneous or nonspontaneous. To explain how this works, three quantities must be introduced: the equilibrium constant, K; the reaction quotient, Q; and the Gibbs Free Energy for a certain reaction, dGr. The equations for K and Q are given below:
where aC corresponds to the activity of C, aD corresponds to the activity of D, etc.
The Gibbs free energy of reaction, dGr, relates to Q and T (temperature) in the following equation:
where dGro is the Gibbs free energy for the reaction at standard conditions.
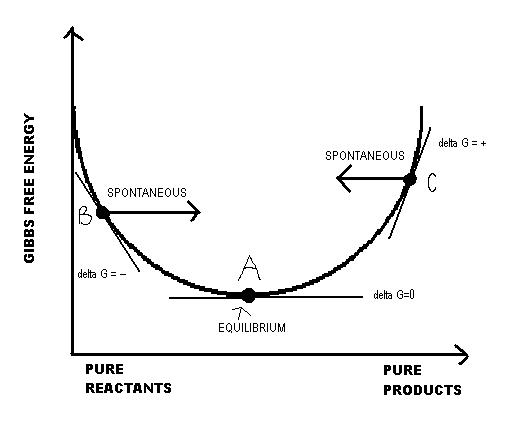
When dGr is negative, the forward reaction is spontaneous and the reverse reaction nonspontaneous. When dGr is positive, the forward reactions is nonspontaneous and the reverse reaction spontaneous. This is due to Q's deviation from K and Le Chatelier's principle at standard temperature. When Q is larger than K, more products are present initially than at equilibrium, according to Le Chatelier's principle, the equilibrium shifts toward the reactants. In other words, the reverse reaction is spontaneous, which corresponds to a positive dGr value. When Q is smaller than K, more reactants are present initially than at equilibrium, the reaction shifts towards the products, thus the forward reaction is spontaneous. This corresponds to a negative dGr value. The relationship between Q and dGr can be represented by the following graph:
At point A, the system is in equilibrium; therefore, Q=K and dGr=0. At point B, Q<K; the forward reaction is therefore spontaneous in order to reach equilibrium, and dGr<0. At point C, Q>K, and thus the reverse reaction is spontaneous in order to reach equilibrium; dGr>0.
Quick Notes
- An equilibrium constant expression includes terms only for reactants and products whose concentrations and/or partial pressures CHANGE during a chemical reaction.
- If an equilibrium system is subjected to a change in temperature, pressure, or concentration of a reacting species, the system will respond with a new equilibrium state.
- When the volume of an equilibrium mix of gases is REDUCED, a net change will occur in the direction that produces FEWER MOLES OF GAS.
- When the volume of an equilibrium mix of gases is INCREASED, a net change will occur in the direction that produces MORE MOLES OF GAS.
Temperature Effects
- INCREASING the temperature will shift the equilibrium in the direction of the ENDOTHERMIC reaction. DECREASING the temperature will cause a shift in the direction of the EXOTHERMIC reaction
- The principal effect of the temperature on the equilibrium is in changing the equilibrium constant.
Catalysts
- A catalyst will change the mechanisms of a reaction to lower the activation energy
- It lowers the activation energy by an equal amount for both the forward and reverse reaction.
ขอขอบคุณข้อมูลจาก
สมัครสมาชิก:
ความคิดเห็น (Atom)













